Home » Products » IVF and Life Science » IVF Equipment & Solutions » Esco Fertility Lab Quality Control
MIRI® GA
Gas and Temperature Validation Unit
Esco Fertility Lab Quality Control
Esco quality control and validation units are technically essential instruments designed to verify the internal environmental conditions of incubators used in Assisted Reproductive Technology (ART). These portable devices provide an independent, high-precision measurement of critical parameters including temperature, CO2, and O2 concentrations. By using an external validation system, embryologists can cross-check the built-in sensors of their incubators against a calibrated reference, ensuring that the micro-environment remains within the strict tolerances required for successful embryo development.
Technically, these validation units are equipped with advanced sensor technology, such as infrared (IR) sensors for CO2 and galvanic or optical sensors for O2 measurement. The temperature is typically monitored using high-accuracy PT1000 sensors or thermistors that can be placed directly inside incubator chambers or attached to heated surfaces. The data captured by these units can be logged in real-time and exported for comprehensive reporting and auditing.
Dual-Channel Gas Concentration Measurement
The validation unit is technically designed to measure both CO2 and O2 levels simultaneously. It utilizes high-grade sensors that can handle the specific gas mixtures used in IVF, such as tri-gas environments. The CO2 is typically measured via an Infrared sensor which is unaffected by humidity, while O2 is measured to ensure the hypoxic conditions (often 5 % are precisely maintained. This dual-channel capability allows for a complete atmospheric profile of the incubator to be established during a single validation session.
High-Precision Temperature Sensing (PT1000)
Temperature is the most critical variable in embryo culture, and the validation unit employs PT1000 Class A sensors to ensure accuracy. These sensors are technically capable of measuring temperature with a resolution of 0.01 C. The unit often supports multiple temperature probes, allowing for the simultaneous monitoring of different shelves or zones within an incubator. This helps in identifying thermal gradients or mapping the temperature uniformity across the entire internal chamber.
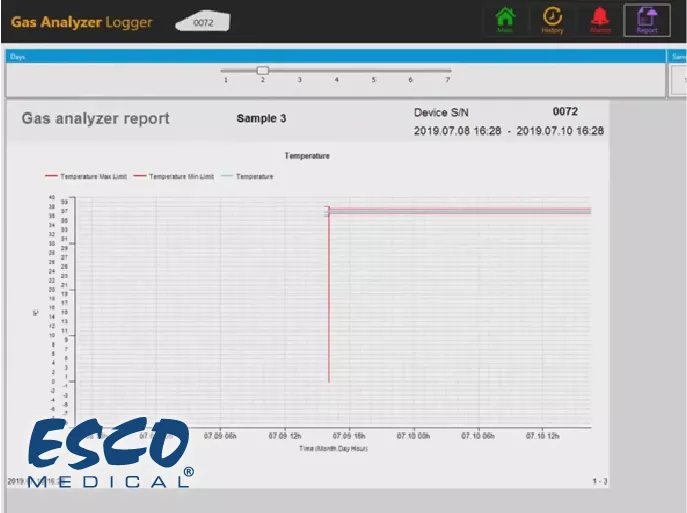
Real-Time Data Logging and Graphing
A key technical feature is the ability to log data over extended periods. The validation unit can record parameters at user-defined intervals, creating a detailed historical record of the incubator's stability. This data can be visualized in real-time as a graph on the unit's display or a connected computer. This is technically significant for observing "recovery times"—how long it takes for the incubator to return to setpoints after a door opening—which is a vital metric for equipment performance.
Portable and Ergonomic Design for Lab Mobility
The validation unit is engineered to be portable, allowing quality control personnel to move easily between different incubators and workstations. Its compact design includes long-lasting battery power and intuitive interfaces. Technically, this mobility ensures that validation can be performed "at-line" without disturbing the laboratory workflow. The unit often comes with specialized sampling kits, including tubing and connectors compatible with the standard sampling ports found on most professional IVF incubators.
Independent Reference for ISO and CAP Compliance
In highly regulated ART environments, relying solely on the incubator’s internal sensors is insufficient for compliance with ISO or CAP (College of American Pathologists) standards. The validation unit serves as an independent reference tool. By having this unit calibrated annually by a certified body, the laboratory establishes a "traceable" chain of measurement. This technical documentation is essential during inspections to prove that the laboratory’s primary equipment is delivering the environment it claims to.
Sampling Pump and Gas Flow Control
To ensure accurate gas readings, the unit incorporates an internal sampling pump. This pump technically draws a precise volume of air from the incubator chamber through the sensors. The flow rate is carefully controlled to prevent creating a vacuum in the incubator or diluting the sample with ambient air. This active sampling method is far more accurate than passive diffusion, as it ensures that the gas reaching the sensors is a true representation of the environment surrounding the culture dishes.
Pressure and Humidity Monitoring Capabilities
Some advanced validation units extend their technical capabilities to include atmospheric pressure and relative humidity (RH) monitoring. Atmospheric pressure can influence gas concentration readings, so integrated pressure sensors allow the unit to automatically compensate for these variations. Monitoring humidity is technically important to ensure that the incubator is maintaining a non-evaporative environment, which prevents the osmolarity of the culture media from rising to toxic levels.
Software Integration for Quality Management
The validation unit is often supported by dedicated software that facilitates data management. Once a validation session is complete, the data can be uploaded to a secure database. The software technically enables the generation of "Validation Certificates" or "Quality Control Reports" that summarize the mean values and deviations. This digital integration streamlines the administrative side of quality control, making it easier to maintain a long-term history of equipment performance for every incubator in the facility.
Read more about Esco Fertility Lab Quality Control