.webp)
Home » Products » Testing Instruments » Pharmaceutical Testing Instruments » Pharma Test Suppository Testing
Suppository Testing
Suppository and pessaries disintegration testers
Pharma Test Suppository Testing
Pharma Test suppository testing instruments are technically engineered to characterize the unique physical and thermal properties of lipid-based dosage forms, such as suppositories and vaginal pessaries. Unlike oral tablets, these dosage forms are designed to melt or disintegrate at body temperature within a physiological environment. Pharma Test provides standardized equipment to measure the disintegration time of these products by subjecting them to controlled temperature and agitation within a water bath.
Furthermore, the galenic range for suppositories includes precise systems for measuring the melting point and the mechanical strength (breaking hardness) of the samples. The melting point is a critical technical parameter, as it must be low enough to allow release in the body but high enough to maintain stability during storage and transport. Similarly, the penetration or breaking test evaluates the structural integrity of the suppository to ensure it can withstand handling without deforming or fracturing.
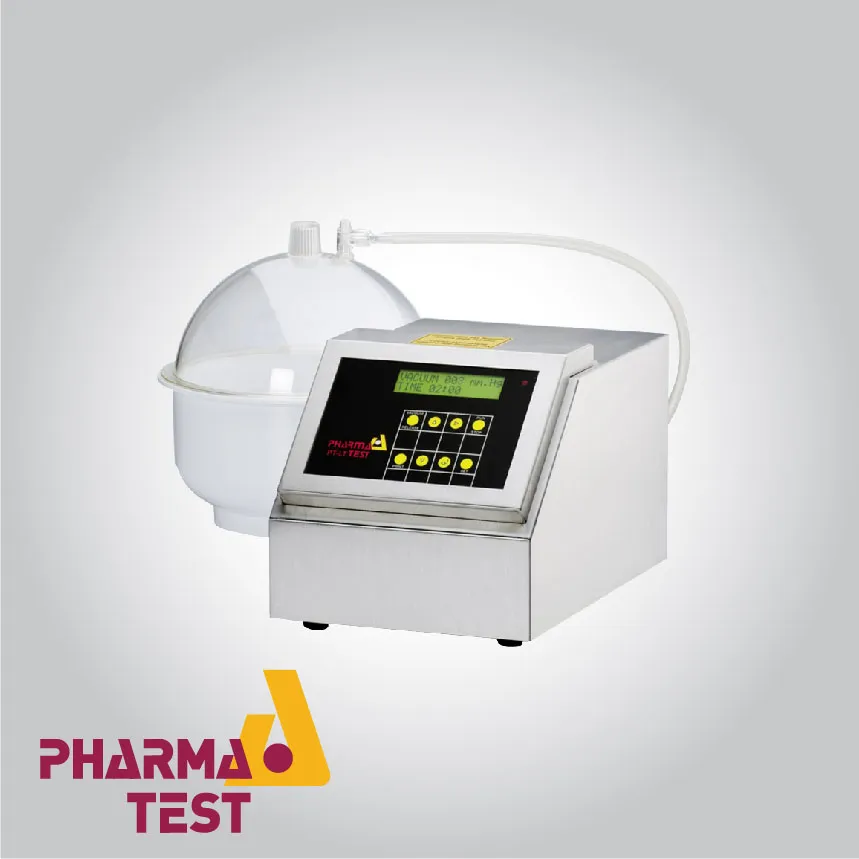
Standardized Disintegration Testing for Suppositories
The disintegration of suppositories is measured using a specialized apparatus that complies with the European Pharmacopoeia. The system features a rotating basket assembly that holds the samples in a water-filled vessel maintained at exactly 37 C The basket is inverted at regular intervals (typically every 10 minutes) to ensure uniform exposure to the medium. This technical procedure is essential for determining the time required for the fatty base to melt or for the hydrophilic base to dissolve, ensuring predictable drug release in the target physiological site.
Melting Point and Softening Time Analysis
Understanding the thermal behavior of a suppository base is critical for product efficacy. Pharma Test instruments facilitate the measurement of the melting point or the "softening time." This process involves placing a suppository in a specialized glass tube submerged in a temperature-controlled water bath. As the temperature rises or remains constant, the time taken for the sample to lose its shape or melt completely is recorded. This technical data is vital for formulating products that remain solid at room temperature but activate precisely at body temperature.
Penetration Resistance and Mechanical Strength
Mechanical stability is a key requirement for suppositories to ensure they can be administered without breaking. Pharma Test offers instruments that measure penetration resistance by applying a specific weight or force to the sample until it yields. This breaking hardness test provides a quantitative value of the suppository's structural integrity. Technically, this helps in optimizing the ratio of waxes and oils in the formulation to achieve the ideal balance between hardness and melting characteristics.
Hydrostatic Pressure and In-Vivo Simulation
Advanced suppository testing sometimes requires the simulation of hydrostatic pressure to better understand how the product behaves within a body cavity. Pharma Test equipment can be configured to apply a controlled pressure environment while the sample is undergoing disintegration or melting analysis. This technical refinement provides a more accurate representation of the physiological forces acting on the dosage form, which is particularly useful for complex or novel lipid-based delivery systems.
Automated Temperature Control and Stability
Consistency in suppository testing is highly dependent on the stability of the water bath temperature. Pharma Test instruments feature high-precision heaters and circulation pumps that maintain the medium within +- 0.5 C of the set point. This level of thermal control is mandatory for pharmacopoeial compliance, as even slight variations in temperature can significantly accelerate or delay the melting process, leading to inaccurate and non-reproducible data.
Specialized Sample Holders and Baskets
To accommodate the diverse shapes and sizes of suppositories and pessaries, Pharma Test provides various sample holders. These are typically manufactured from high-quality stainless steel or chemically resistant plastics to prevent interaction with the medium. The design of these holders ensures that the sample is adequately supported while allowing for maximum surface area contact with the water or buffer solution, ensuring that the disintegration process is not physically hindered during the test.
Large Batch Capacity and Efficiency
For quality control laboratories managing high sample volumes, Pharma Test systems are designed to test multiple units simultaneously. Multi-station testers allow for the independent or synchronized testing of several batches, increasing throughput. This technical configuration ensures that statistical significance can be achieved by testing a representative number of units from a production run, all under identical atmospheric and thermal conditions.
Digital Documentation and Compliance Reporting
Modern galenic testing requires robust documentation for regulatory audits. Pharma Test suppository testers can be integrated with data logging software or printers to record test parameters such as temperature profiles, inversion intervals, and final disintegration times. This technical documentation provides a clear, electronic audit trail that supports 21 CFR Part 11 requirements and ensures that every batch of suppositories meets the specified quality standards before release to the market.
Click here for more information about Pharma Test range of products