Home » Products » Testing Instruments » Pharmaceutical Testing Instruments » Pharma Test Tablet Friability Testing
Tablet Friability Testing
Multiple drum option
Pharma Test Tablet Friability Testing
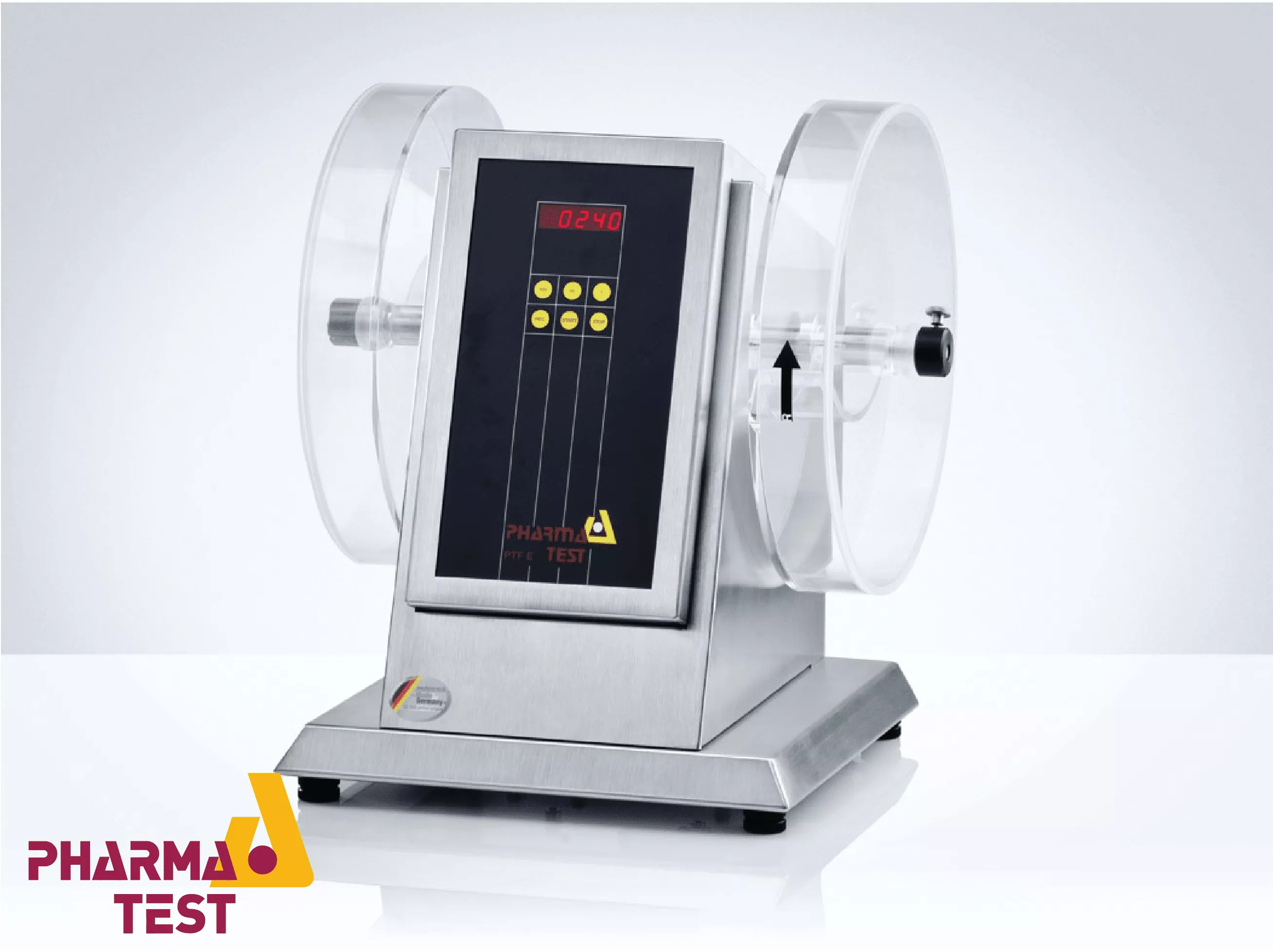
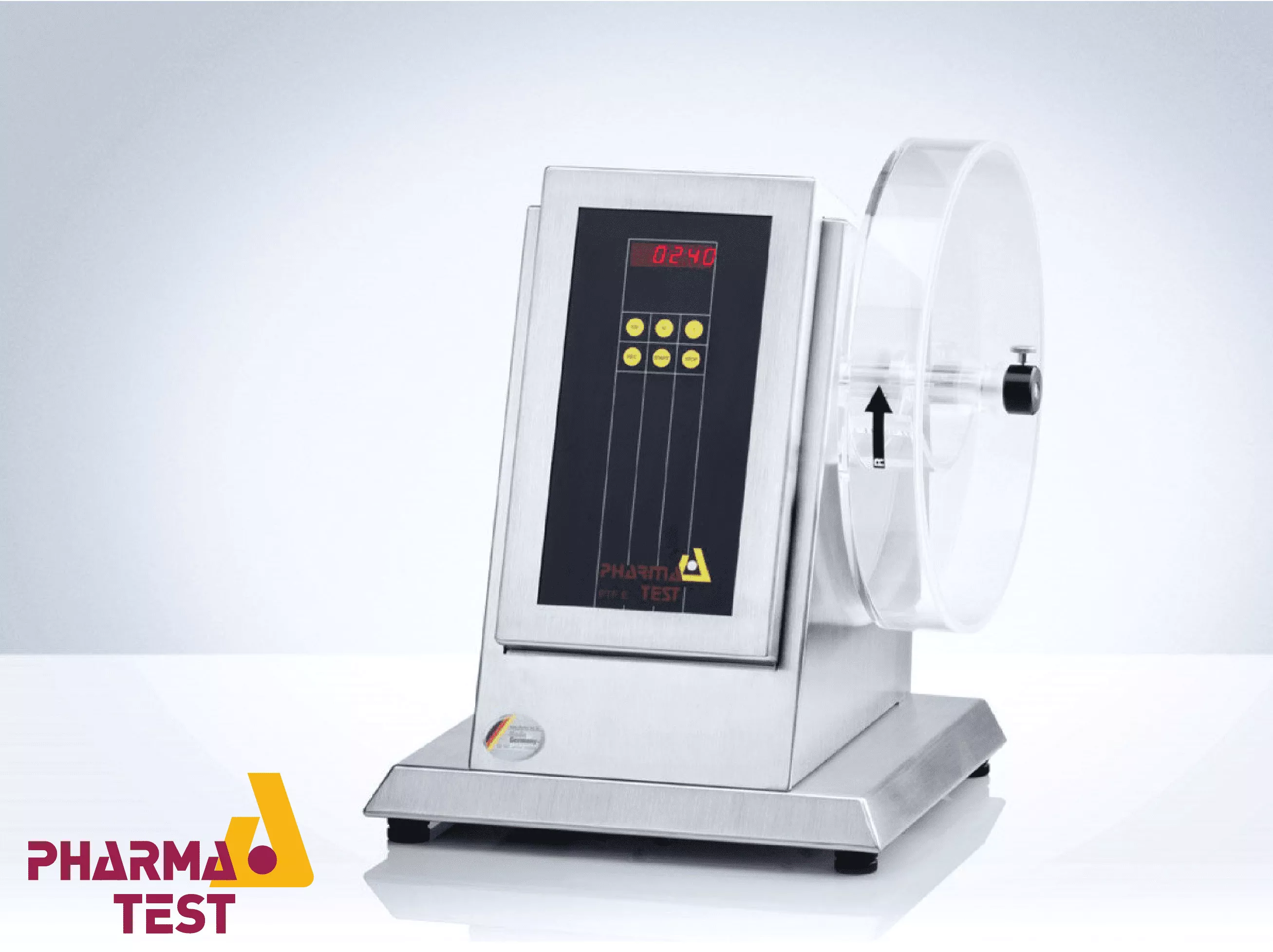
Pharma Test tablet friability testing instruments are technically engineered to evaluate the mechanical durability of uncoated tablets when subjected to physical stress. Friability is a critical pharmaceutical parameter that measures the resistance of a tablet to surface abrasion and chipping during manufacturing, coating, packaging, and shipping. These instruments utilize a standardized rotating drum with an internal scoop that lifts the tablets to a specific height and allows them to fall, simulating the mechanical shocks encountered in a production environment.
The technical design of Pharma Test friability testers strictly adheres to international pharmacopoeial specifications, including USP <1216> and EP <2.9.7>. The systems feature precise motor control to maintain a constant rotational speed (typically 25 RPM) and support automated discharge mechanisms to simplify the testing workflow. Whether configured with single or multiple drums, these instruments provide a controlled environment for testing different batch sizes.
Standardization According to USP and EP Specifications
Pharma Test friability instruments are built to comply with the harmonized methods of global pharmacopoeias. Technically, this requires the use of a "Roche" style drum with an internal diameter of 287 mm and a depth of 38 mm. The internal scoop must have a specific radius that allows the tablets to drop from a height of 156 mm during each rotation. This standardized geometry ensures that the kinetic energy applied to the tablets is identical across all testing facilities, allowing for global data comparison and regulatory validation.
Precise Rotational Speed and Timing Control
Accuracy in friability testing depends on the consistency of the drum's rotation. Pharma Test systems utilize high-precision DC motors that maintain a constant speed, usually set at 25 RPM with a tolerance of +- 1 RPM. The instruments allow the operator to set either a specific number of total rotations (typically 100) or a defined time period. This mechanical precision prevents variations in the physical stress applied to the tablets, which is essential for achieving a valid "pass/fail" result based on the 1% weight loss threshold.
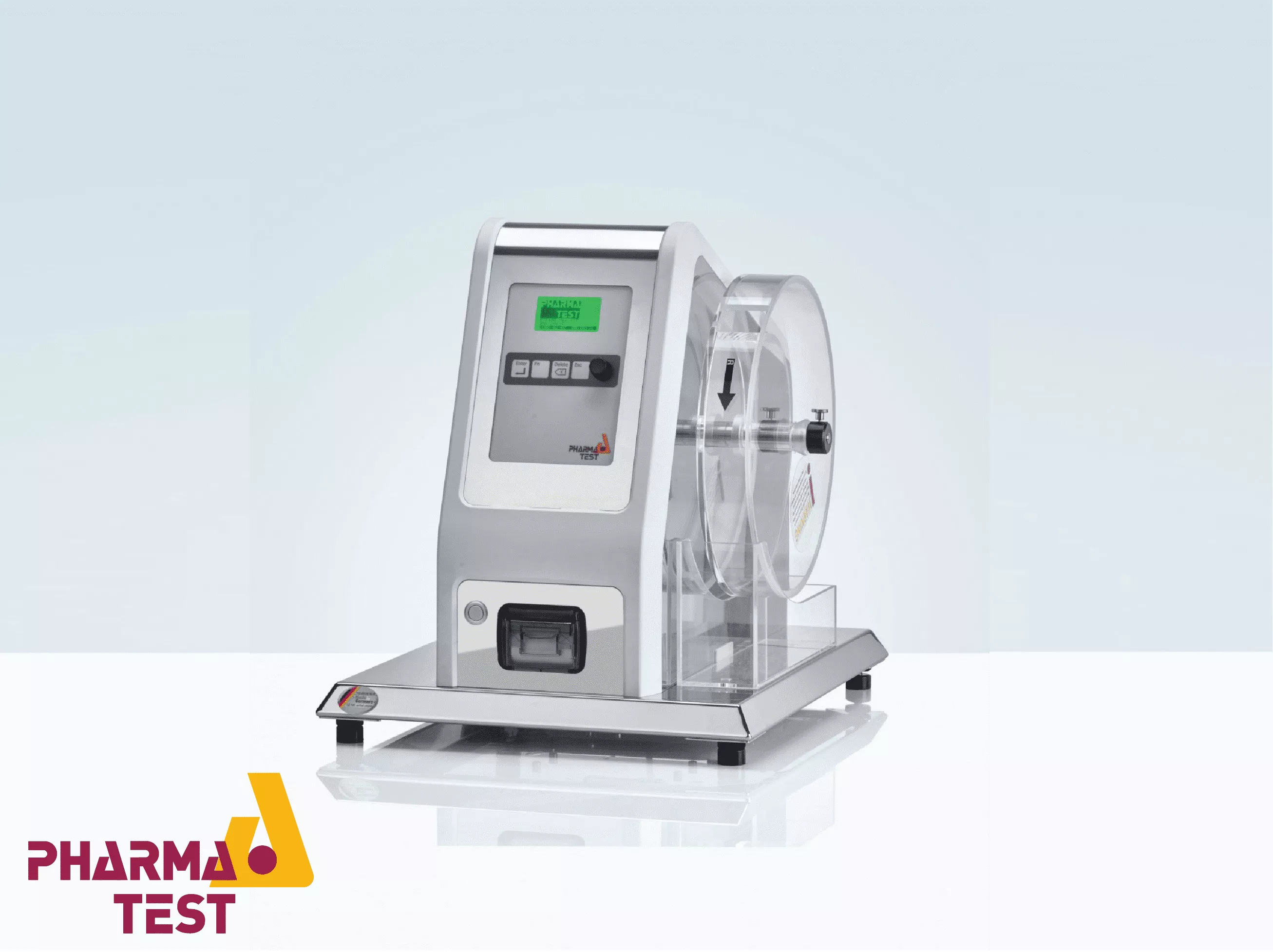
Automated Tablet Discharge and Cleaning
To improve laboratory efficiency and minimize manual handling, Pharma Test friability testers often include an automated discharge function. Upon completion of the test cycle, the drum rotates in the opposite direction, allowing the tablets to be collected in a specialized tray. This technical feature ensures that all particles and tablet fragments are easily removed, preventing cross-contamination between batches. The drums are typically made of anti-static acrylic, which allows for clear visual inspection and easy cleaning.
Single and Multiple Drum Configurations
Pharmaceutical laboratories often need to test different formulations or large batches simultaneously. Pharma Test offers instruments with modular designs that can accommodate one, two, or more drums. Each drum station is driven by a synchronized shaft, ensuring that multi-drum tests are conducted under identical conditions. This configuration is technically advantageous for high-throughput environments where rapid screening of multiple tablet variants is required during formulation development.
Abrasion Testing Capabilities
In addition to standard friability, some Pharma Test instruments support abrasion testing. While friability focuses on the impact of falling, abrasion testing evaluates the surface wear caused by tablets rubbing against each other. Specialized abrasion drums, which may feature internal slats instead of the standard scoop, are used for this purpose. This technical analysis is particularly important for tablets that will undergo subsequent film coating, where surface smoothness and integrity are paramount.
Tilting Mechanism for Large Tablets
For larger tablets or those with a specific geometry that might not fall correctly in a vertical drum, Pharma Test systems include a 10 C tilting mechanism. This technical adjustment ensures that the tablets tumble in a consistent manner rather than sliding along the drum wall. By tilting the entire drive assembly, the instrument ensures that the mechanical stress remains uniform even for non-standard dosage forms, fulfilling specific pharmacopoeial requirements for larger specimens.
Integrated Calculation and Balance Connection
To streamline the analytical process, Pharma Test systems can be interfaced with analytical balances. The operator weighs the tablets before and after the test, and the data is transmitted directly to the instrument’s processor. The software then automatically calculates the percentage of weight loss. This eliminates manual calculation errors and provides a digital readout of the friability index, which is a key component of the final quality control report and audit trail.
Data Integrity and Documentation Standards
Following the requirements of 21 CFR Part 11, Pharma Test friability testers are designed to provide robust data documentation. The instruments can be connected to printers to produce detailed reports including the date, time, number of rotations, and the calculated friability result. This documentation serves as a validated record for regulatory inspections, proving that the tablets meet the required physical standards for durability and structural stability before being released for distribution.
Read more about Pharma Test Tablet Friability Testing