.webp)
Home » Products » Microbiology Laboratory Solutions » Sterilizers and Washers » Fedegari Stand-alone Lab Sterilizers
Stand-alone High-Capacity Steam Sterilizers
Pharmaceutical Grade Performance
Fedegari Stand-alone Lab Sterilizers
The operational core is managed by the proprietary DCS20 or Thema4Lab process controller, a highly advanced system pre-validated according to GAMP5 guidelines, which is crucial for regulated sectors. This controller provides a configurable, multi-user environment with up to 30 custom cycles, offering unparalleled precision in managing complex sterilization recipes. Its robust software supports demanding compliance requirements, enabling end-to-end data traceability and detailed cycle report generation, which fundamentally simplifies the administrative burden of validation and regulatory auditing.
Flexibility in application is paramount, with options like double vertical sliding doors to facilitate pass-through functionality between different contamination control zones, maximizing workflow efficiency in classified environments. Furthermore, specialized configurations, including a single or double stainless steel sealing flange (BIOSEAL), make these sterilizers suitable for rigorous Bio-Safety Levels (BSL3 and BSL4) in research centers and hospitals, providing an essential layer of safety and isolation against high-pathogen risks.
The modular design principle ensures Configurability, allowing laboratories with evolving needs to easily adapt the system through a wide portfolio of optional kits and chamber sizes, mitigating the necessity for total equipment replacement as processing requirements change. This adaptability extends the equipment's lifecycle and maximizes the initial investment.
Process Reproducibility is guaranteed by the utilization of the proprietary DCS20/Thema4Lab control system, which maintains tight control over temperature and pressure parameters throughout the cycle, eliminating the risk of failed runs or compromised product integrity often associated with generic, less validated controls. The system’s adherence to GAMP5 standards assures that the sterilization process will yield consistent, predictable results every single time, which is essential for regulated applications.
Maintaining Containment Integrity in high-risk environments, such as BSL3 and BSL4 laboratories, is achieved through specialized design options like the stainless steel sealing flange, or BIOSEAL. This feature ensures absolute segregation between the clean and contaminated areas, preventing microbial escape and effectively solving critical cross-contamination safety concerns.
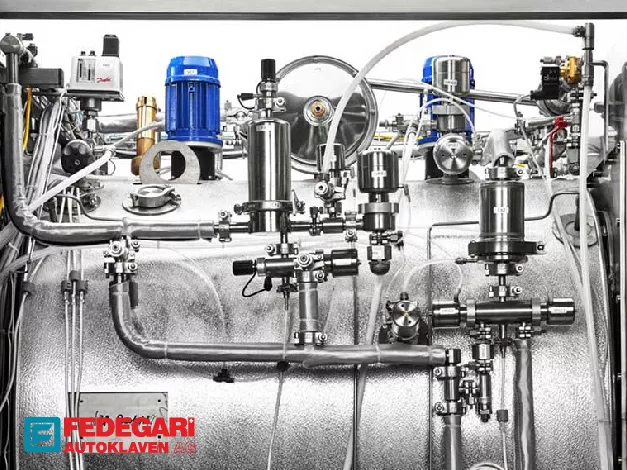
The integrated internal heat-exchanger system is the cornerstone of Utility Optimization, strategically reusing the heat from steam condensate to pre-heat incoming water before steam generation. This intelligent thermal recovery mechanism substantially reduces the overall required thermal energy, leading to measurable decreases in long-term power and water consumption expenses.
Designed for constrained laboratory settings, the use of pneumatically activated, vertical sliding doors contributes to a maximized Ergonomic Footprint. This unique door mechanism requires minimal lateral space for operation, allowing the sterilizer to be positioned efficiently in high-traffic or compact areas without obstructing adjacent equipment or walkways.
Regulatory compliance is significantly streamlined by embedded support for Data Traceability, with the validated control software providing audit-ready cycle documentation and capabilities for adherence to stringent data management mandates. This critical function assures that every sterilization run is verifiable and defensible for quality assurance and official regulatory scrutiny.
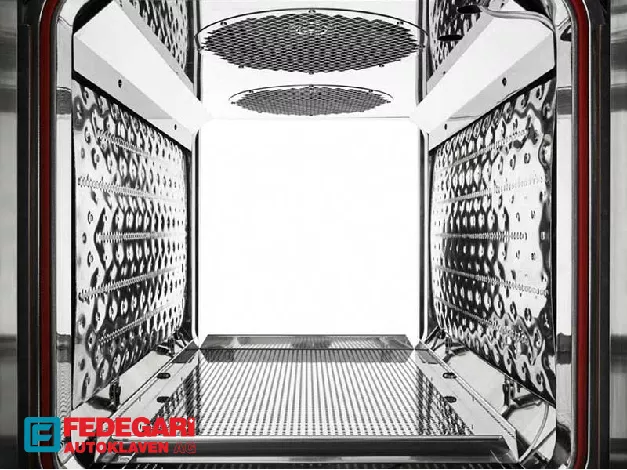
The chamber and critical wetted components are constructed from high-grade AISI 316L stainless steel, providing unmatched Material Longevity and superior corrosion resistance, particularly when sterilizing harsh media or chemical waste. This commitment to quality material selection reduces the frequency of replacement parts and extends the machine's operational uptime.
Accessibility to all crucial hydraulic and pneumatic components is streamlined via full and easy access to the technical area from the front and lateral sides, significantly improving Maintenance Accessibility. This design philosophy ensures serviceability is uncomplicated and swift, minimizing equipment downtime and maximizing lab productivity.
The capacity to handle highly varied and complex loads, from large volume parenterals to wrapped tools and laboratory waste, highlights the Load Versatility of these units. Internal plates can be optioned for final drying purposes, broadening the sterilizer’s utility across a diverse range of laboratory functions and material requirements.
Read more about Fedegari Stand-alone Lab Sterilizers











.webp)
.webp)