Home » Products » Operational Environment Solutions » Bio-Decontamination Systems » Bioquell Biodecontamination Systems
Biodecontamination Systems
Rapid, adaptable and productive
Bioquell Biodecontamination Systems
Bioquell specializes in providing tailored, effective solutions for bio-decontamination within the Pharmaceuticals and Life Sciences sectors, focusing on risk reduction for critical environments. The core of their technical offering is a unique Hydrogen Peroxide Vapor (HPV) technology. This process involves distributing a uniform layer of hydrogen peroxide vapor onto all exposed surfaces within a sealed area. Crucially, this technology is validated to achieve a high-level 6-log sporicidal kill, which is the required standard for sterilization in regulated industries. Bioquell systems are designed to support every step of sensitive processes, from whole-room bio-decontamination to specialized isolators integrated into manufacturing lines, ensuring the maintenance of aseptic manufacturing and compounding conditions.
The applications of Bioquell's technology span critical areas including pharmaceutical manufacturing, research and laboratory spaces, biotechnology and drug development, and high-risk gene and cell therapy. The systems are utilized to maintain the integrity of working and testing environments, helping clients comply with strict regulations concerning aseptic processing and environmental controls. Whether through mobile and built-in systems or a Rapid Bio-Decontamination Service for business continuity needs like facility restart or remediation, Bioquell provides validated, automated decontamination cycles. This ensures increased productivity, fast validation, and definitive contamination reduction.
Hydrogen Peroxide Vapor Technology for Sporicidal Kill
Bioquell's core technological offering is the distinct Hydrogen Peroxide Vapor (HPV) technology. This method is engineered to provide a 6-log sporicidal kill, which signifies the decontamination of one million biological indicators. The process is characterized by the distribution of a uniform layer of hydrogen peroxide vapor across all surfaces exposed within the treated environment. The vapor, unlike aerosolized or UV systems, ensures comprehensive coverage and penetration, targeting even complex geometries. The technology is utilized with an EPA-registered Hydrogen Peroxide Sterilant, confirming its efficacy and trustworthiness by regulatory agencies for high-level surface disinfection and sterilization in critical scientific applications.
Systems for Aseptic Manufacturing Conditions
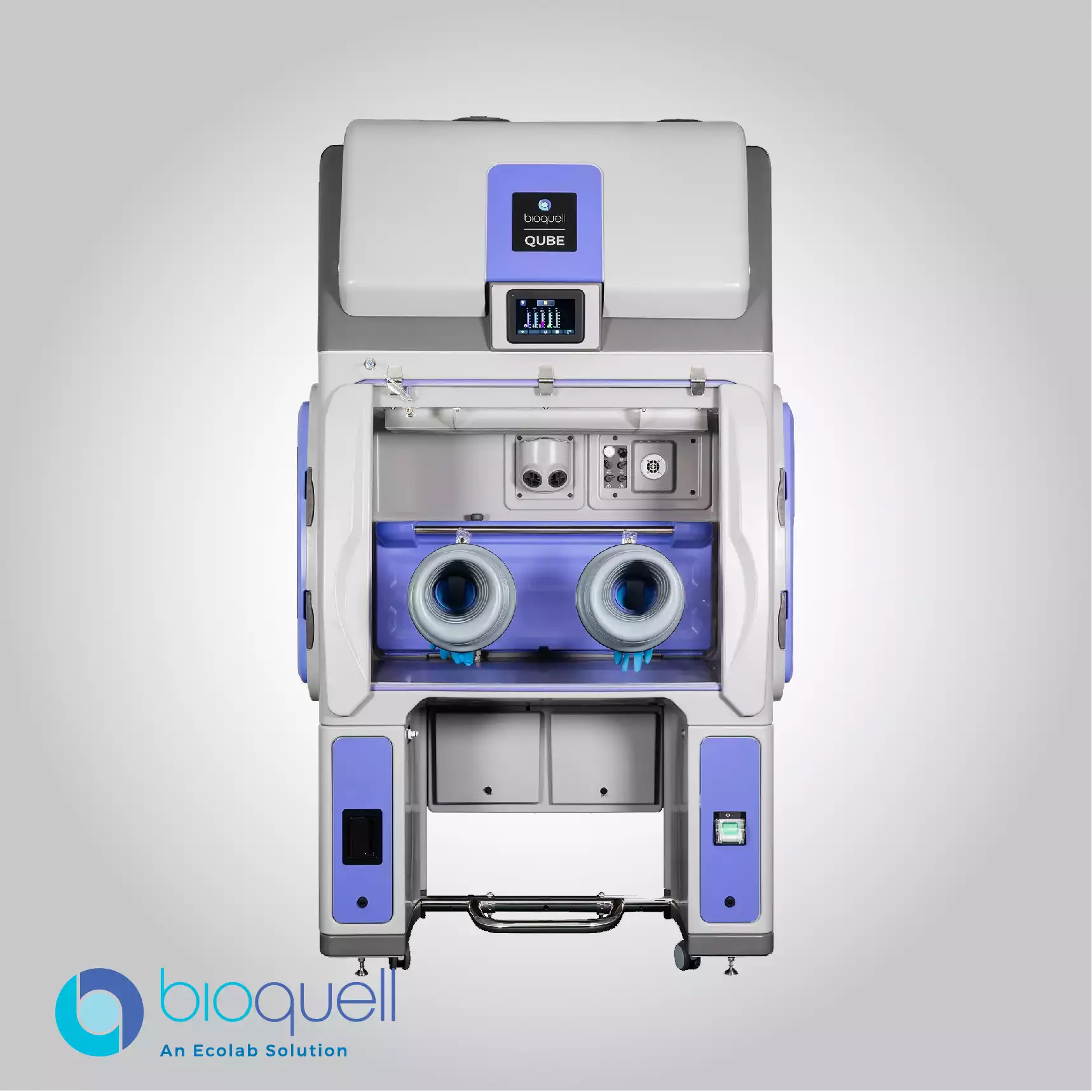
Bioquell provides integrated solutions specifically for maintaining aseptic manufacturing and compounding conditions within pharmaceutical environments. The systems range from equipment designed for whole-room bio-decontamination to specialized isolators engineered for critical processing areas. These isolators function as modular aseptic processing workstations, optimized for sensitive tasks such as sterility testing and the preparation of cytotoxic preparations. By integrating the proprietary vapor technology directly, these systems ensure that the working environment remains free of microbial contamination, thereby supporting the strict, continual application of environmental controls required for final drug and product integrity.
Controlled Decontamination for Research and Laboratories
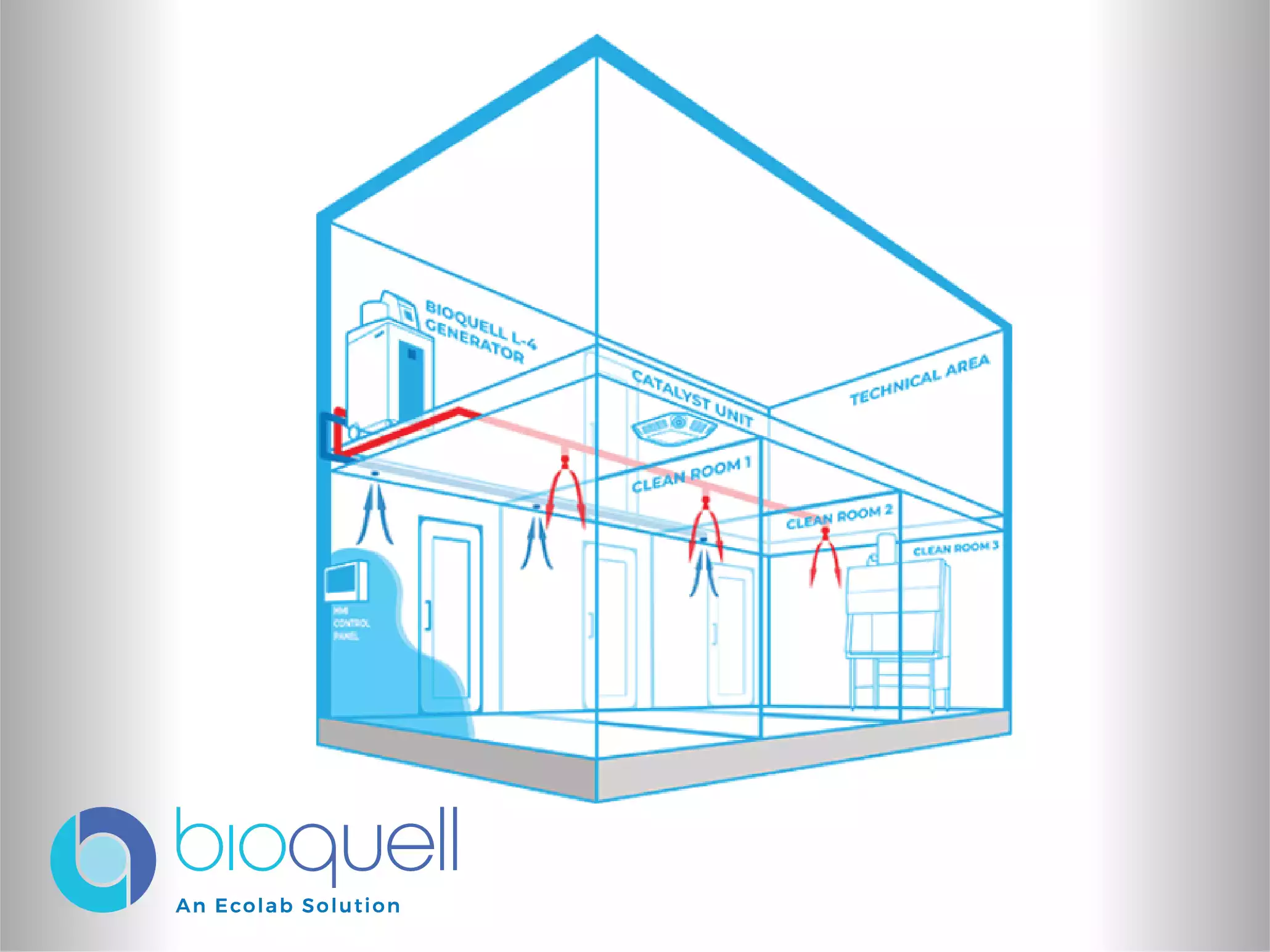
For the research and laboratory environment, where biological safety is paramount, Bioquell offers adaptable and flexible bio-decontamination solutions. These solutions are available in both mobile and built-in system configurations, catering to research facilities of all sizes, large and small. The primary objective is to allow research institutions to reduce the risk of contamination at any stage of the process. The deployment of automated, validated decontamination cycles ensures that lab and research spaces, including those utilized for viral or bacterial vector work, maintain the required bio-security and cleanliness standards with high productivity.
Specialized Systems for Pass-Throughs and Cleanrooms
To optimize workflow and decontamination processes within restricted areas, dedicated systems are engineered for fixed installation. These wall-mounted systems are technically ideal for environments such as pass-throughs, which are critical transition zones used to maintain differential pressures between clean areas, and for use within biological cleanrooms. The systems facilitate fast validation and promote increased productivity through an automated, validated decontamination cycle. Their application ensures effective bio-security and regulatory compliance in specific areas where continuous material transfer and stringent environmental control are necessary.
Validation and Regulatory Trust
Decontamination efficacy is supported by comprehensive Validation processes, which are key to gaining regulatory trust globally. Bioquell utilizes its proprietary sterilant, which is EPA-registered and BPR-approved (Biocidal Products Regulation), confirming its status as a trusted agent by regulatory agencies worldwide. This formal registration and approval provide scientific proof of the product's ability to achieve the specified sporicidal performance. The robust validation process helps facilities maintain regulatory compliance and assures users of the integrity and consistency of the decontamination process, crucial for all life sciences applications.
Biosecurity for Gene and Cell Therapy Environments
The development and manufacturing of Gene and Cell Therapy products present unique challenges due to the extremely high risks associated with microbial contamination. Bioquell addresses this demand by offering a range of rapid and flexible biodecontamination solutions specifically tailored to create the ideal aseptic environment required at every step of this high-risk process. By rapidly and definitively neutralizing contaminants, these systems allow developers to focus on advancing therapies and treatments, safe in the knowledge that the manufacturing and processing spaces meet the highest possible standards for aseptic conditions and environmental control integrity.
Rapid Service for Business Continuity and Remediation
Beyond permanent installations, a Rapid Bio-Decontamination Service is offered to support business continuity and emergency situations. This service is technically critical for a variety of applications, including facility commissioning, end of campaign and facility restart procedures, and handling remediation needs. It is also utilized for emergency response and as a planned component of a disaster recovery plan. The service employs the core HPV technology to rapidly decontaminate affected spaces, such as laboratories or cleanrooms, allowing quick return to operational status with verified 6-log sporicidal performance.
Comparison to Other Decontamination Methods
Bioquell's approach is often contrasted with alternative methods such as UV light or generic aerosolized systems. The technical distinction lies in the ability of the Hydrogen Peroxide Vapor to ensure uniform coverage on all exposed surfaces, overcoming the limitations of UV light's line-of-sight requirement and the non-sporicidal nature or poor penetration of many aerosolized agents. This vaporization technique guarantees that the sterilant reaches complex and shadowed areas, achieving the necessary 6-log kill required for genuine sterilization, offering a technically superior solution for comprehensive contamination control across the Life Sciences sector.
Read More About Bioquell Products Here.