Qube Isolator
with Bioquell’s Hydrogen Peroxide Vapor technology.
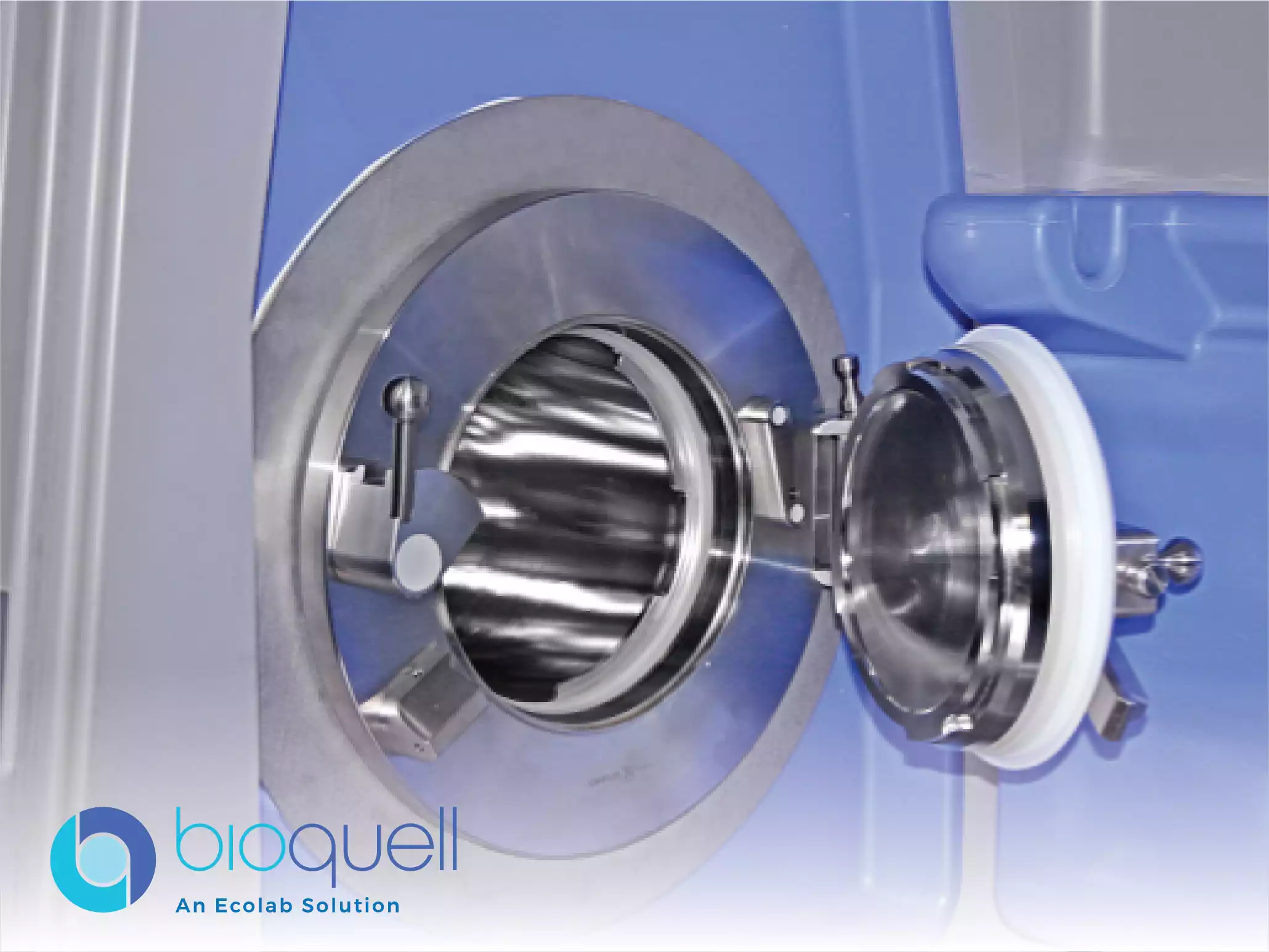
Bioquell Qube Isolator
The Bioquell Qube Isolator is a highly configurable, modular aseptic processing workstation engineered specifically for stringent pharmaceutical and life sciences applications. Its defining technical feature is the integration of the proprietary Hydrogen Peroxide Vapor technology, which ensures reliable and repeatable biodecontamination results. The system is designed to meet the critical requirements for maintaining an ISO 5/EU Grade A environment, which is paramount for sensitive procedures like sterility testing, gene and cell therapy manipulation, and cytotoxic preparations. With its optimized decontamination cycles and integrated control systems, the Qube aims to provide an assured, productive working environment that adheres to strict regulatory standards, including those outlined in GMP Annex 1.
Technically, the Qube is built for flexibility and efficiency, featuring configurations of up to three chambers and two material transfer areas to accommodate complex workflows. It offers dual capabilities for maintaining both positive and negative pressure regimes, which can be easily switched via the touchscreen interface without requiring physical component changes. Furthermore, the isolator boasts optimized cycle times for rapid bio-decontamination and allows for the decontamination of materials in one chamber while work proceeds in another, significantly enhancing workflow productivity. Its construction, utilizing tough and hard-wearing materials like polypropylene and stainless steel for the working surface, facilitates ease of installation and validation.
Integrated Hydrogen Peroxide Vapor Decontamination
The Qube Isolator features built-in Hydrogen Peroxide Vapor technology for achieving reliable and repeatable decontamination results. This integrated system performs rapid bio-decontamination cycles, achieving an assured 6-log kill on all exposed surfaces within the chambers, a benchmark standard for sterilization in pharmaceutical operations. The vapor generation and aeration process are optimized due to the isolator’s unique build, resulting in faster cycle times compared to conventional sterilization methods. The system's design eliminates the need for external HVAC connections for positive pressure use and is managed through an easy-to-use touch-screen interface.
Maintaining ISO 5 and EU Grade A Environment
The core function of the Qube is to maintain a highly controlled, aseptic working environment. It is validated to consistently provide an ISO 5/EU Grade A environment, ensuring the lowest possible level of microbial and particulate contamination. This high level of cleanliness is supported by the implementation of unidirectional airflow within the chamber, in accordance with the strict requirements of GMP Annex 1. This ensures that air moves consistently and smoothly across the critical working space, sweeping away any potential contaminants and supporting the integrity of highly sensitive operations.
Configurable Chamber and Material Transfer Areas
The Qube is engineered to be highly adaptable to diverse laboratory workflows. Its modular design allows for configurations of up to three chambers, each typically featuring two gloves, alongside the option of incorporating up to two separate material transfer areas. This configurable layout allows users to segment their process steps, ensuring dedicated areas for material entry, primary manipulation, and exit, all while maintaining full containment. This modularity is key to meeting varied operational needs and optimizing the flow of materials and products through the aseptic process.
Pressure Control and Safety Regimes
A critical technical feature of the Qube is its ability to operate under switchable positive and negative pressure capabilities. This dual functionality is essential for managing different levels of risk; positive pressure is utilized to protect the product inside the isolator from external contamination, while negative pressure is used for handling hazardous materials, such as cytotoxic preparations, to protect the operator. The system includes programmed leak tests for both pressure regimes and allows for pressure switching simply via the touch screen, with no additional component changes required for safety.
Advanced Environmental Monitoring Capabilities
The isolator supports rigorous quality control and assurance through four levels of environmental monitoring. These options range from manual systems to fully integrated particle monitoring, accommodating both viable and nonviable particle counting. This comprehensive monitoring suite provides users with continuous, real-time data on the aseptic conditions within the chamber, essential for meeting regulatory audit requirements. This capability supports the necessary environmental controls to ensure product safety and allows for setting alarms for temperature conditions inside the chamber.
Features Optimizing Sterility Testing Workflow
The Qube Isolator is specifically optimized to enhance the sterility testing process. It offers the efficient option of integrating up to two dedicated sterility test pumps. This integration simplifies operations and frees up valuable workspace that would otherwise be occupied by external equipment. Furthermore, the ability to rapidly decontaminate materials in the transfer area or a secondary chamber while the main sterility testing procedure is underway significantly improves process productivity and ensures that the testing environment remains consistently aseptic.
Rapid Installation and Validation Process
The physical design and construction materials, notably the tough and hard-wearing polypropylene body, contribute to the system's rapid deployment. The Qube can be installed and fully validated in a timeframe as quickly as sixteen weeks from order. The physical installation is minimally invasive, typically requiring only a standard electrical outlet and no connection to HVAC for positive pressure applications. Furthermore, the unit is designed to fit through standard door frames, allowing it to be built directly in the designated area. The validation process includes full documentation for Installation, Operational, and Performance Qualification (IQ, OQ, and PQ).
Enhancing Long-Term System Utility and Flexibility
The Qube Isolator is designed with foresight for evolving process needs, allowing for certain upgrades to be easily added after installation. This includes integrating accessories such as a glove tester, a particle counter, or an active air sampling device, which can enhance the system's capabilities affordably. The chamber also provides an option for a floor-mounted one-inch triclover port in each chamber, enabling users to securely introduce liquid lines or cables into the working area without compromising containment integrity. This design focus on post-installation flexibility maximizes the long-term utility of the aseptic workspace.
Read More About Bioquell Products Here.





.webp)