Home » Products » Testing Instruments » Pharmaceutical Testing Instruments » Pharma Test Ampoule Testing
Ampoule Testing
Control the quality during production of empty ampoules
Pharma Test Ampoule Testing
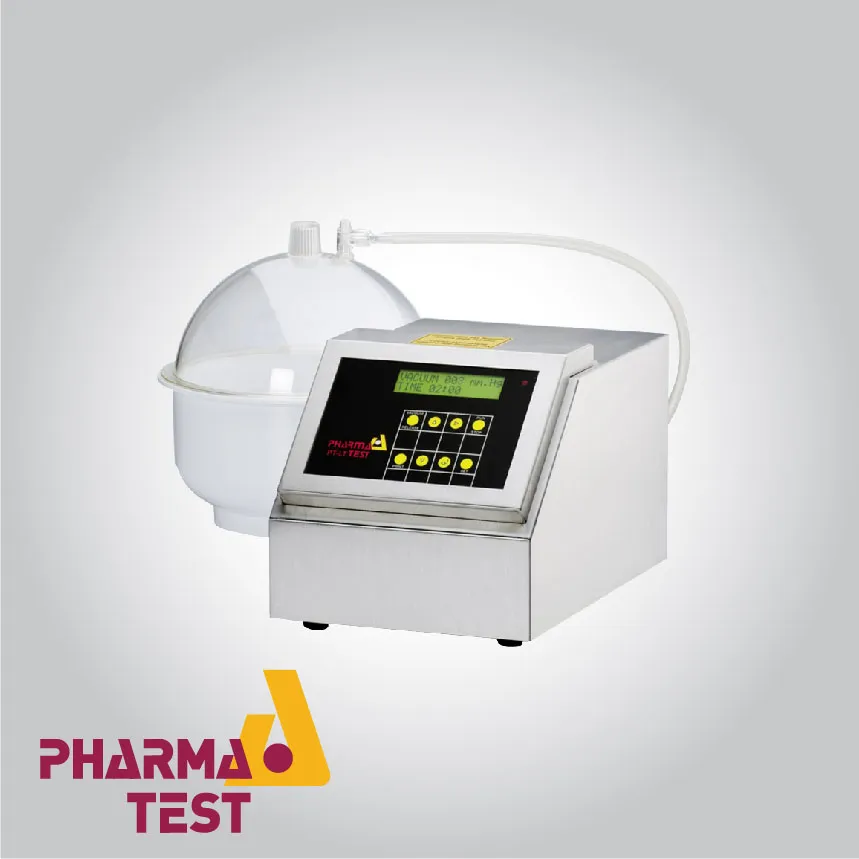
Pharma Test offers a specialized suite of instruments designed to verify the physical integrity and safety of ampoules and other small-volume parenteral containers. Testing ampoules is a critical technical requirement in the pharmaceutical industry because these containers hold sterile liquids intended for direct injection into the human body. Any breach in the seal or a hairline crack in the glass can lead to microbial contamination or degradation of the active pharmaceutical ingredient. Pharma Test equipment focuses on standardized leak testing procedures, often utilizing vacuum-induced methods to identify compromised containers that might otherwise pass visual inspection.
Technically, the validation of ampoule integrity involves rigorous stress testing under controlled atmospheric conditions. Pharma Test instruments facilitate the "blue dye" test or methylene blue test, where ampoules are submerged in a dye solution within a vacuum chamber. When a vacuum is applied and subsequently released, any leak in the ampoule causes the dye to be drawn into the container, providing a clear and undeniable visual confirmation of failure.
Principles of Vacuum Leak Detection
Leak testing for ampoules is fundamentally based on the principle of differential pressure. Pharma Test instruments create a controlled vacuum environment within a testing chamber where the ampoules are submerged in a tracer fluid. As the air pressure inside the chamber decreases, any air trapped within a leaking ampoule is forced out. Upon the restoration of atmospheric pressure, the surrounding fluid is forced into the ampoule through the same defect. This technical procedure is the industry standard for identifying microscopic leaks that cannot be detected through simple atmospheric observation.
The Blue Dye Integrity Test Method
The methylene blue dye test is the most widely recognized technical method for assessing the seal integrity of glass ampoules. Within the Pharma Test apparatus, a specific concentration of dye is used to ensure high visibility. The process is strictly timed and the vacuum levels are precisely controlled to ensure that even the smallest capillaries or cracks are penetrated by the tracer. This method provides a qualitative but highly reliable "pass/fail" result, ensuring that every container in a validated batch is hermetically sealed.
Glass Stress and Thermal Stability Testing
Ampoules are often subjected to extreme temperatures during sterilization processes like autoclaving. Pharma Test equipment helps evaluate how these glass containers respond to thermal and mechanical stress. If the glass has internal tensions or if the sealing process has made the tip brittle, the ampoule may fail during subsequent handling. Technical testing ensures that the glass quality and the sealing technique are robust enough to withstand the pressure changes and heat required for maintaining a sterile product.
Validation of Hermetic Sealing Processes
The sealing of an ampoule is a high-temperature process where the glass is melted to close the container. Pharma Test instruments serve as the primary validation tool for the sealing machinery. By testing samples from the production line, technicians can determine if the flames are correctly adjusted or if the "pull-seal" mechanism is creating consistent wall thickness at the tip. This technical feedback loop is essential for minimizing waste and preventing the distribution of sub-standard injectable products.
Compliance with Global Pharmacopoeial Standards
Pharmaceutical manufacturers must adhere to strict guidelines set by the USP, EP, and JP regarding the integrity of parenteral packaging. Pharma Test equipment is technically aligned with these international standards, providing the documented proof required for regulatory audits. The instruments ensure that the testing parameters—such as vacuum duration and pressure depth—are consistent with pharmacopoeial requirements, allowing for global method transfer and standardized quality reporting.
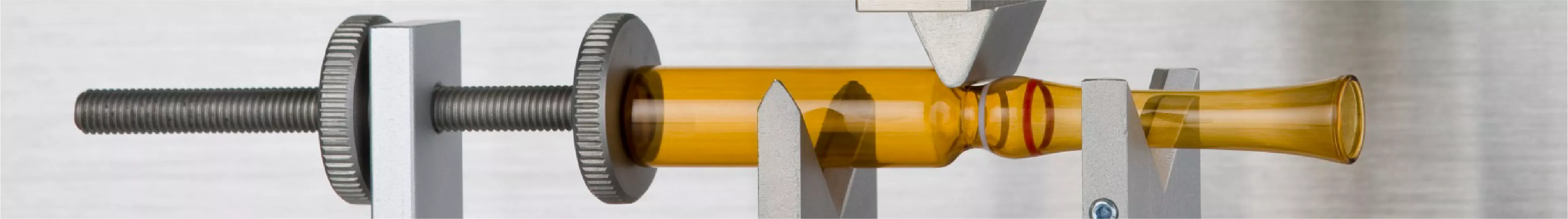
Mechanical Resistance and Break-Force Analysis
While the seal must be perfect, the ampoule must also be easy for healthcare professionals to open. Technical testing of the "snap-off" or "one-point cut" (OPC) areas is vital. Pharma Test solutions can be used to monitor the physical force required to break the ampoule neck. This ensures that the glass breaks cleanly without shattering or creating glass splinters (shards) that could contaminate the medication or injure the user, balancing structural integrity with practical usability.
Large Batch Testing and Reproducibility
In a production environment, testing a single ampoule is insufficient; reproducibility across large batches is required. Pharma Test chambers are designed to hold multiple ampoules simultaneously in specialized racks. This technical configuration ensures that every ampoule in the test group is subjected to the exact same vacuum profile and submersion depth. This uniformity is critical for statistical process control and for ensuring that the results of the leak test are representative of the entire production run.
Data Integrity and Operational Safety
Modern pharmaceutical testing requires more than just a visual result; it requires data integrity. The control systems for Pharma Test leak testers are designed to provide clear, digital readouts of the vacuum levels and cycle times. This prevents operator bias and ensures that the testing protocols are followed strictly every time. Furthermore, the instruments are built with high-grade stainless steel and chemically resistant materials to withstand constant contact with dye solutions and cleaning agents, ensuring long-term operational reliability in a lab setting.
Click here for more information about Pharma Test range of products