Home » Products » Chromatography/Mass Spectrometry » Gas Chromatograph-Mass Spectrometry » Shimadzu GC-MS Software
GC-MS Software
Advanced GC-MS Data Processing and Lab Management Software
Shimadzu GC-MS Software
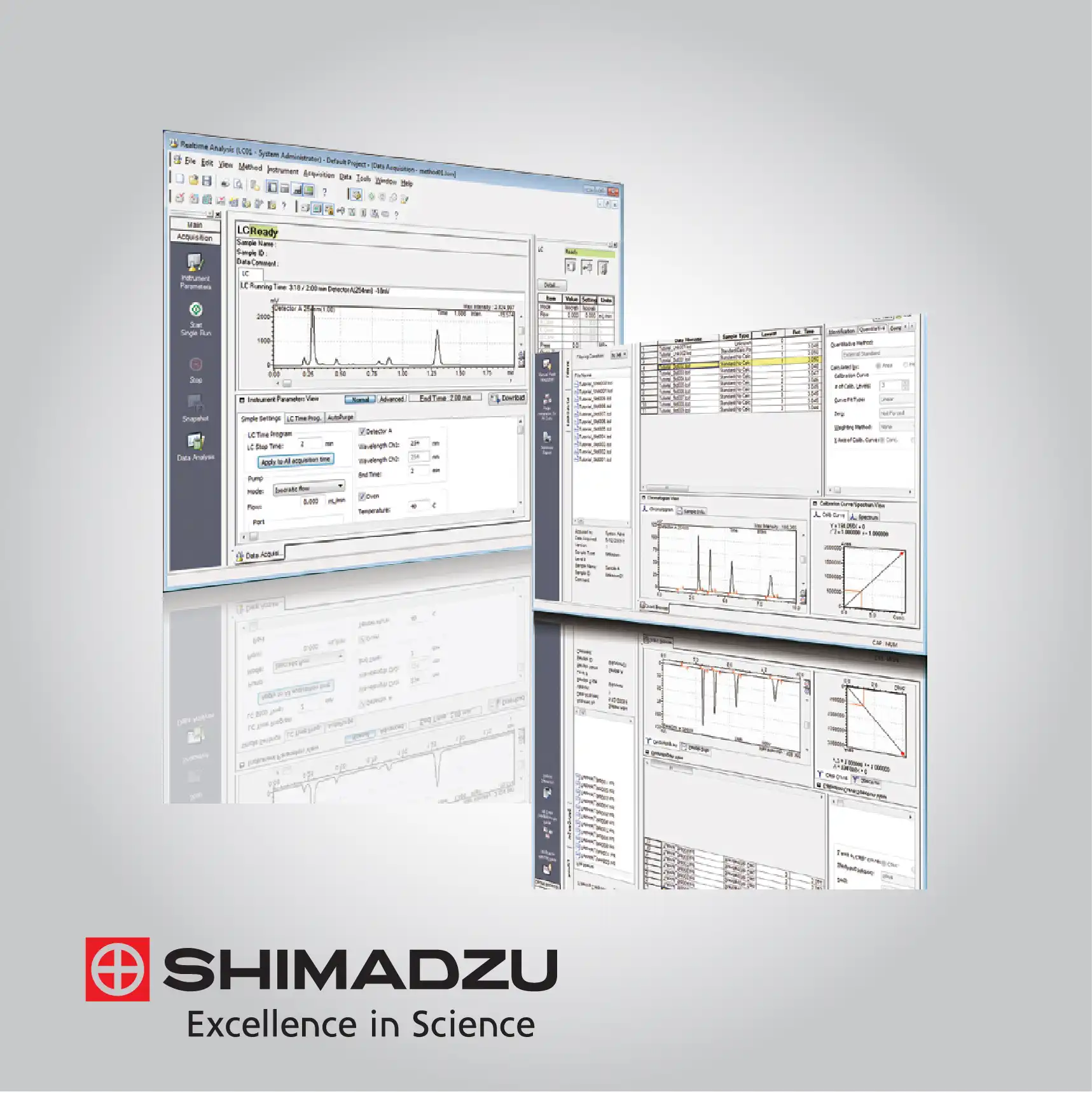
A core technical advantage of Shimadzu’s GC-MS software is its deep integration of AI and networked data management to ensure total compliance with global standards like FDA 21 CFR Part 11. The "Peakintelligence" AI engine utilizes deep learning to perform peak integration with the expertise of a veteran analyst, drastically reducing manual correction time. Furthermore, the software supports a client-server architecture via LabSolutions CS, which centralizes all analytical data into a secure database.
This ensures complete data integrity through robust audit trails, user management, and electronic signatures. The inclusion of "LabSolutions Direct" further enhances flexibility by allowing analysts to monitor real-time instrument status and results from remote devices, ensuring that laboratory operations remain continuous and transparent regardless of the operator's physical location.
AI-Powered Peak Integration (Peakintelligence)
Peakintelligence for GCMS is a transformative AI algorithm developed through deep learning of thousands of chromatograms integrated by experienced analysts. Technically, it eliminates the need for manual parameter settings such as slope and width, which often vary between operators. The AI can accurately detect peaks even in complex matrices with low signal-to-noise ratios or difficult-to-allocate baselines. This reduces manual peak integration time to approximately 1/4 of the conventional time, ensuring consistent and objective results across the entire laboratory.
Smart MRM and Automated Method Creation
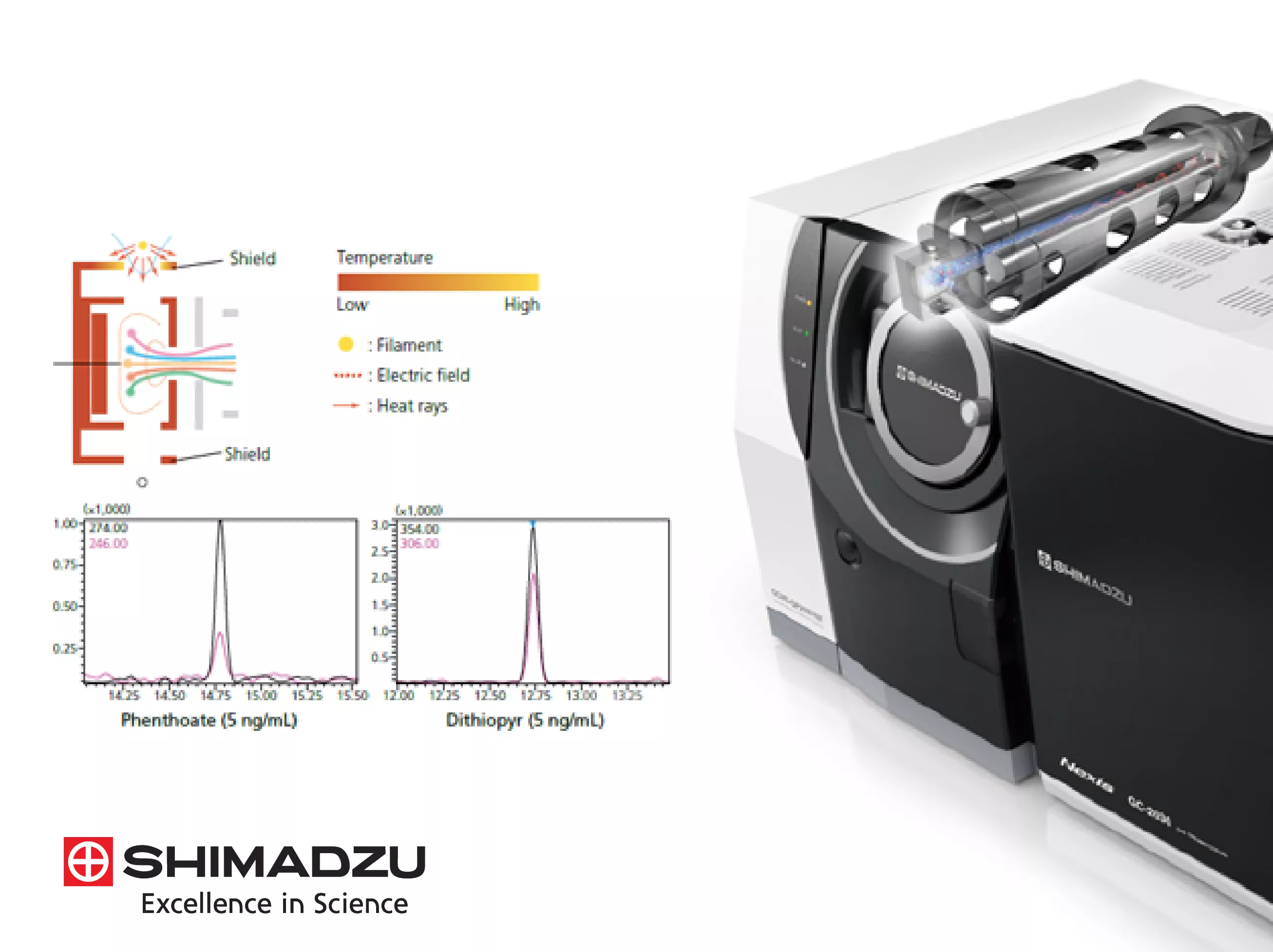
The Smart MRM technology technically automates the complex task of creating Multiple Reaction Monitoring (MRM) methods for triple quadrupole systems. By utilizing the "Smart Database" series, the software automatically sets optimized transitions and collision energies for hundreds of compounds. It aligns these transitions with the estimated retention times provided by the AART function, ensuring that data is acquired with maximum sensitivity only when the target components are eluting. This maximizes the number of compounds that can be analyzed in a single run without losing data quality.
Automatic Adjustment of Retention Time (AART)
The AART function is a critical technical tool for maintaining method consistency. It calculates the retention indices of target compounds based on the analysis of an n-alkane standard and then automatically adjusts the retention times in the compound table. This prevents the need for manual updates after column maintenance or when moving a method between different instruments. Technically, it ensures that peak identification windows are always correctly positioned, maintaining the reliability of qualitative and quantitative results over the long term.
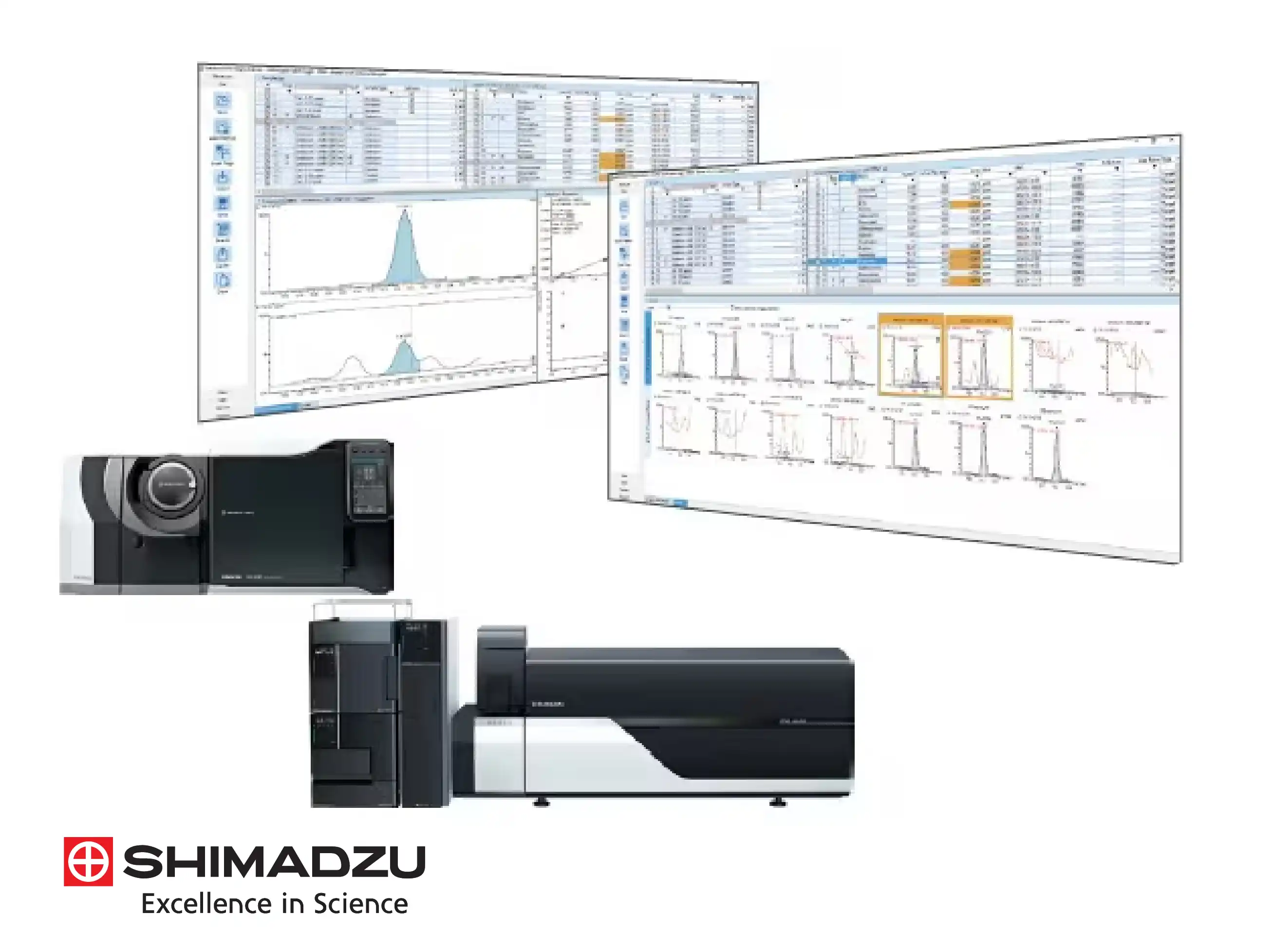
LabSolutions Insight for High-Throughput Review
LabSolutions Insight provides a specialized technical environment for reviewing massive amounts of multi-component data. It features a customizable "Quantitation Browser" that allows analysts to compare chromatograms, calibration curves, and results for multiple samples side-by-side. The software includes over 75 QA/QC flagging criteria, such as recovery rates and reference ion ratios, which automatically highlight out-of-spec results. This streamlined workflow is essential for laboratories performing large-scale screening in food safety or environmental testing.
Secure Networked Data Management (LabSolutions CS)
For laboratories requiring strict data integrity, LabSolutions CS provides a client-server architecture that centralizes data from GC-MS, LC-MS, and other instruments. Technically, all raw data, method files, and batch schedules are stored in a secure server database, preventing unauthorized deletion or overwriting. Analysts can access the data and perform post-run analysis from any networked PC, while the system maintains a comprehensive audit trail that logs every action taken, ensuring full compliance with ER/ES guidelines.
Extensive "Smart Database" Support
Shimadzu provides a wide range of application-specific databases, including those for pesticides, forensic toxins, environmental pollutants, and metabolites. These databases technically store not only transitions and collision energies but also retention indices and mass spectra. When combined with the Smart MRM or Smart SIM functions, these databases allow users to start analysis immediately without the tedious process of searching for fragments or optimizing collision conditions, significantly accelerating laboratory setup for new applications.
Remote Monitoring with LabSolutions Direct
LabSolutions Direct is a technical solution that connects smart devices—such as smartphones and tablets—to the LabSolutions workstation. Users can remotely check the status of an ongoing analysis, monitor the real-time chromatogram, and view the estimated completion time. This functionality allows for "analysis while walking," enabling researchers to manage their laboratory instruments from the office or other areas. It also provides a safe way to monitor potentially hazardous analyses from a distance, enhancing laboratory safety and efficiency.
Compliance and Data Integrity Features
To meet FDA 21 CFR Part 11 requirements, the software incorporates advanced security features including multi-level user access control, password complexity management, and electronic signatures. Technically, the system prevents the alteration of raw data files and maintains "Check Sum" values to detect any tampering. The automated "Report Set" function ensures that all relevant data, including audit trails and method parameters, are bundled together in a single, unalterable PDF report, simplifying the regulatory review process.
Click here for more information about Shimadzu range of products
Related Products
Ask Us A Question
CONTACT US
Taawon for Laboratory & Scientific Supplies
Taawon Group © 2026 - All Rights Reserved
We use cookies to optimize site functionality and give you the best possible experience






.webp)
.webp)